
Eat Your: Chicken
When committing to a fitness style diet plan, one of the first things you may be told is to start eating chicken. The baked-chicken-for-dinner-every-night stereotype


Triplet oxygen O2 is the oxygen we know as atmospheric oxygen. This is more properly represented as O22•because it has two unpaired electrons. When excited by a photon of light it can become singlet oxygen (O2*). There are other means to turn triplet oxygen into singlet oxygen besides light, though this is the best known mechanism.
| O22• | + | light | → | O2• |
Figure 1- Atmospheric oxygen activated by a photon of light to become singlet oxygen
Singlet oxygen can give an electron to become superoxide (O2–).
| O2* + e– | → | •O2– | -0.33V | (reduction reaction) |
Figure 2- Singlet oxygen gaining an electron to become superoxide.
Singlet oxygen is actually a weak electron donor which gives up an electron, but once this happens it becomes a powerful oxidizing agent, superoxide.
Oxidation is rightly named after oxygen, and there are many forms of oxygen that can become radicals. These are thought of as a group- Reactive Oxygen Species (ROS). Some of the reactions outlined here show the explicit reactants and products that become the different ROS. Figure 3 below, taken from the work of Paul Wood. (Wood, 1988)[1] reveals the many steps between oxygen and its daughter radicals.

Figure 3- Potential diagram for oxygen at pH 7. The units are in volts and the value in parentheses is for 1 mol of O2 ∙l-1 as a standard state, instead if 104 Pa of O2.
Other reducing potentials are available as “Standard Reducing Potential” charts also known as “The Activity Series” and a great number of these are available on the internet. One excellent example is from “Hyperphysics” by C.R. Nave from Georgia State University (Nave C. R., 2014)[2]. Standard potentials will be taken from the two sources above for the rest of this discussion.
To understand the chemical equations and half reactions and how these are used in chemistry to determine the spontaneous nature of a reaction and also to determine how much voltage a reaction can produce, I recommend the Hyperphysics description of how these reactions get sorted out into their chemical equations. (Nave C. , 2014)[3]
1 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1149288/pdf/biochemj00228-0281.pdf
2 http://hyperphysics.phy-astr.gsu.edu/hbase/tables/electpot.html#c1
3 http://hyperphysics.phy-astr.gsu.edu/hbase/chemical/electrode.html#c1
The Body’s Enzymatic Detoxification for Superoxide- Superoxide Dismutase (SOD)
Superoxide is detoxified by Superoxide Dismutase (SOD). These reactions happen within an enzyme, so no direct measurement is possible. Below are all of the chemical interactions in equations, then what follows are a breakdown of the individual components involved in those reactions.
| Cu2+-SOD + •O2− | → | Cu+-SOD + O2 |
| Cu+-SOD + •O2− + 2H+ | → | Cu2+-SOD + H2O2 |
Figure 4 – Reactions of copper superoxide dismutase to detoxify superoxide to hydrogen peroxide and oxygen.
The reactions can be thought of as their non-enzymatic component reactions. Copper (I) goes to Copper (II) and back.
| Cu2+ + e– → Cu+ | +0.15V |
| Cu+ → Cu2+ + e– | -0.15V |
Figure 5 – Standard reducing potentials for the gain or loss of an electron between copper (I) and copper (II).
The catalytic power of copper ions and the forward and back reactions are coupled to superoxide, which gains an electron to become molecular oxygen.
| •O2– → O2* + e– | +0.33V | (Oxidation reaction) |
Figure 6 – The reaction that makes superoxide lose an electron to become singlet oxygen.
All of the above reactions are coupled together with the catalytic help of copper and zinc ions (Zn-Cu SOD has a zinc and a copper ion in adjacent positions in its active site). The mechanical movement of the enzyme coordinates the individual reactions above and allows superoxide to take an electron. This allows its transformation to hydrogen peroxide.
| •O2– + 2H+ + e– → H2O2 | +0.089V |
Figure 7- Superoxide becomes a stable molecule of hydrogen peroxide, which is safely transportable in the bloodstream.
The Body’s Enzymatic Detoxification for Hydrogen Peroxide- Catalase
When the body deals with hydrogen peroxide in a productive manner, it converts it to oxygen and water with the help of the enzyme catalase.
| 2H2O2 → 2H2O + O2 | +1.349V |
Figure 8- Hydrogen peroxide can be degraded to oxygen and water using the enzymatic power of catalase.
Other Oxidizing Agents and Radicals that are in Common Use
The equations and reactive species above are not commonly encountered in everyday life. There are oxidizing agents that we are familiar with that do generate reactive oxygen species (ROS). One potent source of ROS commonly used is the OXY- type materials such as OxiClean or Oxy-Boost. These are forms of hydrogen peroxide that have been stabilized with a salt. In the case of OxiClean sodium carbonate (washing soda) is combined with hydrogen peroxide to make sodium carbonate peroxyhydrate. When this is dissolved in water, the hydrogen peroxide is liberated from the washing soda and they have two effects. The washing soda is a powerful agent for increasing pH (alkaline) and the hydrogen peroxide can oxidize organic molecules like the brown pigments left after a stain has set in fabric.
Bleach is another very commonly used oxidizing agent. It has troubling products that make it unsuitable for use when foods are going to be prepared on a surface. Bleach can leave behind chlorinated organic compounds such as trihalomethanes, which form when chlorine reacts with organic materials; an example of trihalomethane is chloroform, which is a well-known carcinogen. Bleach also has the problem of releasing a toxic oxidizing agent- chlorine gas (Cl2) and chloramines if mixed with ammonia. Bleach also releases hydrogen peroxide, which becomes part of the oxidizing action of bleach. Bleach is sodium hypochlorite NaClO. The many reactions that can come from it are illustrated below in figure 9.
First the hydrochloric acid is formed:
| NaOCl + H2O | → | NaOH + HClO |
| H2O + HClO | → | HCl + H2O2 |
Figure 9- The reactions of bleach with water to form hydrochloric acid and hydrogen peroxide.
If bleach is mixed with acid, the result is chlorine gas (Figure 10). If mixed with ammonia it can produce chloramine (figure 11). Both of these gasses are very toxic and are especially dangerous since they are released as a vapor and may be inhaled:
| NaOCl + 2HCl | → | Cl2(g) + NaCl + H2O |
Figure 10- The reactions of bleach with acids to form chlorine gas
| 2NH3 + Cl2(g) | → | 2NH2Cl |
Figure 11- The reactions of bleach with ammonia to form toxic chloramine gas.
If ammonia is present in excess (which it may or may not be, depending upon your mixture), it may form the toxic and potentially explosive liquid hydrazine.
While impure hydrazine does not tend to explode, it’s still toxic, plus the heat liberated by the exothermic reaction means that it can boil and spray hot, toxic liquid.
| 2NH3 + NaOCl | → | N2H4 + NaCl + H2O |
Figure 12- The reactions to form hydrazine with bleach and ammonia.
The oxidizing agents we use have many uses, but the commonality between them is that they destroy organic molecules. In our mitochondria, the action of oxygen upon glucose leads to the production of water and carbon dioxide (figure 13). This is a useful, harnessed reaction that provides us with the energy we need to live and move. This is very similar to the reactions that occur inside the flame from burning wood, since wood is composed mostly of glucose polymers (cellulose).
| C6H12O6 + 6O2 | → | 6CO2 + 6H2O |
Figure 13- The reactions of glucose and oxygen to produce carbon dioxide and water.
When there is a useful function for the reaction inside mitochondria the effect is beneficial- much like the flame from a campfire is useful if it is cooking food. When the reactions occur outside the mitochondria, the result is destruction, much like the fire that rages through the forest is destructive, even though it is chemically similar to the campfire that cooked a delicious meal.
This is the problem of antioxidants- how to have them extinguish the fires outside the allowed area, while leaving the “hearth” of the mitochondria untouched. We have found that the insulation provided by the cell membrane as well as the mitochondrial membrane make a preferential flow of electrons toward free radicals outside the mitochondria. This is much like the way that rain cannot fall down a chimney with a chimney cap. Fires outside the chimney can be extinguished by the rain, while fires that are sheltered are unaffected. With Heliopatch, we bring the (figurative) rain of electrons with exquisite targeting.
An Alternate Detoxification of Hydrogen Peroxide Using Magnesium Metal
When magnesium is connected to a person, hydrogen peroxide can follow a different path to detoxification using H+ (acid) from the body and electrons from the magnesium. Magnesium has a very potent voltage for its reduction that is higher than any biological molecule. Magnesium has a standard reducing potential of -2.37 volts. In the equation below, the magnesium is being oxidized, so the equation is reversed and the sign of the voltage of the reaction becomes positive. This is a half-reaction which is paired up with an electron accepting half-reaction and the two voltages are summed together to indicate the total voltage of the reaction. Voltage determines how far electrons can flow from one point to another. This is one of the keys to the potency of magnesium, it can act at a distance from the radical it neutralizes.
| H2O2(aq) + 2 H+(aq) + 2e– | → | 2 H2O(l) | +1.77V |
| Mg(s) | → | Mg2+(aq) + 2e– | +2.37V |
| = | +4.14V (spontaneous at a distance due to voltage) |
Figure 14- The reactions between hydrogen peroxide and magnesium’s electrons.
This reaction consumes two H+ (acid) with H2O2 and turns them into water with the help of magnesium’s electrons. This leads to an alkalizing effect in the body wherever hydrogen peroxide is made.
The Body Has No Enzymatic Detoxification for the Hydroxyl Radical
Hydrogen peroxide can also be converted into the most potent radical in biology, the hydroxyl radical (OH•) with the help of free iron or other transition metals.
| Fe3+ + H2O2 | → | Fe2+ + O2• + 2H+ | -0.89V |
| Fe2+ + H2O2 | → | Fe3+ + OH• + OH− | +0.38V |
Figure 15- The Fenton’s reagent reaction that produces both superoxide (O2•) and hydroxyl radicals (OH•).
There are no enzymatic means to detoxify hydroxyl radicals as can be done with superoxide or hydrogen peroxide. These radicals are so rapidly produced and react instantly with other biomolecules such that even the fastest enzymes cannot stop them. For a hydroxyl radical, the only previously known defense are small molecule antioxidants- but these only function if they are in close proximity. The voltage available from biomolecules is not great enough to act at a distance. One well-known example of an antioxidant that can neutralize almost any radical is ascorbate (Vitamin C), which reorganizes its bonds after oxidation to become a stable ascorbyl radical. The ascorbyl radical can then be regenerated or excreted. In figure 16 below, X could be any radical. (Soderberg, 2015)[4]
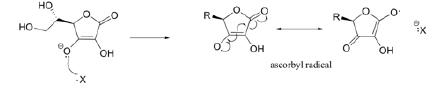
Figure 16- The reaction of ascorbate with a free radical to form the ascorbyl radical, which stabilizes by bond rearrangement.
Elemental Hydrogen- a Weak Antioxidant with Great Potential
Another example of a small antioxidant capable of neutralizing hydroxyl radicals is elemental hydrogen (H2). Hydrogen is an excellent antioxidant because of its mobility, but this also means that a large amount of the hydrogen introduced into the body is lost through the skin and breath. Hydrogen is not a very potent antioxidant, so it can scavenge only hydroxyl radicals, and has to be within close proximity to the hydroxyl when it forms in order to neutralize it. Hydroxyl radicals exist for only microseconds, so the opportunity to stop this radical is very limited by the proximity hydrogen has to it when it appears or it will react with some other part of the body’s infrastructure such as DNA, enzymes or proteins. The product of the reaction is water. A very well-written discussion of Hydrogen’s potential in medicine can be found in a recent review of hydrogen in medicine (Ohta, 2011)[1].
| 2OH•(aq) + 2e– | → | 2OH–(aq) | +2.38V | |
| H2(g) | → | 2H+(aq) + 2e– | 0.0V | |
| H2(g) | → | 2H2O(aq) | = | +2.38V |
Figure 17- The product of the reaction of a hydroxyl radical and hydrogen gas is water.
Magnesium versus Hydroxyl Radicals- Neutralization at a Distance and Alkalization
In contrast to the relatively weak antioxidant that hydrogen is, magnesium has 2.37 volts more energy behind its donation of electrons. This means that the electrons emitted by magnesium can travel through the body’s conductive tissues to an area where a radical is demanding electrons. This means that unlike the weak antioxidant hydrogen, the magnesium can act at a distance and with “lightning fast” speed. This means that instead of transport, depletion and removal of products, the electrons go into the tissue where the inflammation exists and give a naked electron that is not limited by factors such as blood flow, energy requiring active transport and diffusion.
| 2OH•(aq) + 2e– | → | 2OH–(aq) | +2.38V (inside the body) | |
| Mg(s) | → | Mg2+(aq) + 2e– | +2.37V (within the patch) | |
| → | 2H2O(aq) | = | +4.75V |
Figure 18- The reaction between electrons from magnesium and two hydroxyl radicals produces hydroxide (OH–).
4http://chemwiki.ucdavis.edu/Core/Organic_Chemistry/Organic_Chemistry_With_a_Biological_Emphasis/Chapter_17%3A_Radical_reactions/Section_17.2%3A_Radical_chain_reactions
5 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3257754/
The product of this reaction inside the body is hydroxide (OH–). The magnesium ions on the exterior of the body can migrate into the body, providing a valuable source of magnesium nutrient.
Hydroxide is the opposite of acid (H+) and raises pH and creates an alkaline environment. This alkalizing effect has long been used by athletes and trainers to enhance performance and endurance. The burning sensation of exerted muscles is caused by acids produced inside them; lactic acid is the most well-known because this is a direct product of anaerobic metabolism of sugars caused by oxygen depletion.
| H+ + OH– | → | H2O |
Figure 19- The reaction between base (OH–) and acid (H+) to produce water. The balance of these determines the pH of a solution.
When acid and base collide, the result is water (H2O). Water requires no further transport or detoxification unlike an ascorbyl radical, and would contribute to the hydration of the tissues where it is formed by an acid-base neutralization.
Most other means for creating alkalinity depend upon ingestion of distasteful and sometimes dangerous solutions of bicarbonate that cause disruption of the stomach’s normal acidity and can cause malabsorption of nutrients, since stomach acid is, after all, useful to the digestive process.
This means of creating alkalinity is quite different. The chemistry occurs spontaneously at a distance from the source (Heliopatch) and creates alkalinity within the tissues that are stressed most. This is the ideal matchup of the need for alkalinity in the tissues that produce the most hydroxyl radicals, which also happen to be the most acidified tissues and the ones where the alkalinity will produce the greatest benefits. This unique chemistry turns the body’s greatest problem– hydroxyl radicals, into hydroxide, which is the solution for a different problem– acidity. This is much like the tactic of tricking your enemy’s army to attack another of your enemies.
Works Cited
Nave, C. (2014). Discussion of Standard Electrode Potentials. Retrieved 7 21, 2015, from Hyperphysics:
http://hyperphysics.phy-astr.gsu.edu/hbase/chemical/electrode.html#c1
Nave, C. R. (2014). Table of Standard Electrode Potentials. Retrieved 7 21, 2015, from Hyperphysics:
http://hyperphysics.phy-astr.gsu.edu/hbase/tables/electpot.html
Ohta, S. (2011). Recent Progress Toward Hydrogen Medicine: Potential of Molecular Hydrogen for Preventive and Therapeutic Applications. Curr Pharm Des., 2241–2252. Retrieved from
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3257754/
Soderberg, T. (2015). Section 17.2: Radical chain reactions. Retrieved 7 21, 2015, from Organic Chemistry With a Biological Emphasis: http://chemwiki.ucdavis.edu/Organic_Chemistry/Organic_Chemistry_With_a_Biological_Emphasis/Chapter_17%3A_Radical_reactions/Section_17.2%3A_Radical_chain_reactions
Wood, P. M. (1988). The Potential Diagram for Oxygen at pH 7. Biochem J, 287-289. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1149288/pdf/biochemj00228-0281.pdf

When committing to a fitness style diet plan, one of the first things you may be told is to start eating chicken. The baked-chicken-for-dinner-every-night stereotype

Every athlete has their idols. The person they saw on TV as a kid and thought, “That’s who I want to be.” For

