
Eat Your: Chicken
When committing to a fitness style diet plan, one of the first things you may be told is to start eating chicken. The baked-chicken-for-dinner-every-night stereotype

When we talk about the naked electron, it sounds simple, but electrons are hard to pin down. Describing what an electron is can be quite difficult because they are the very definition of ephemeral. Electrons have no mass, or at least their mass is unmeasurable. We can only “see” them by their effects upon other things. When they move they create a magnetic field. We can also observe what happens chemically when they are taken away, a process called oxidation. Likewise, we can see the result when they are added, which is known as reduction. Redox (reduction/oxidation) reactions are at the heart of almost every life process. Every reduction reaction is accompanied by an equivalent oxidation reaction; these are two sides of the same coin.
These chemistry terms can be difficult because they can be counterintuitive. For instance, reduction is an odd term; after all, didn’t we add an electron? It is easier when you think of the charge of an electron being negative. In algebra, adding a negative number is the same as subtraction, so the result is essentially subtraction when an electron is added and the overall charge of a molecule goes down. Add an electron to iron (III) (Fe+3) and you get iron (II) (Fe+2); adding an electron reduces the charge but it is interesting to notice that in this case we did not create a negative ion.
When we talk about antioxidants, we are really talking about reducing agents. Reducing agents are chemicals that are good electron donors, and most of the time these are molecules with lengthy electron sharing structures called conjugated systems. Conjugation can be seen in molecules with single and double bonds in alternation and these long conjugation systems are also what create many natural pigments we find in colorful fruits and vegetables.
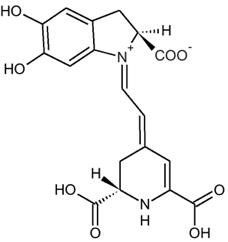
Figure 1 – Betanidin from beets, an example of a conjugated molecule
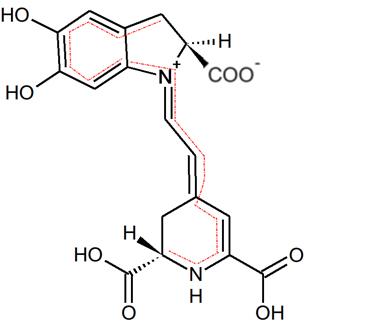
Figure 2 – Betanidin from beets with the conjugated system traced in red. This conjugation provides both the red color and the potent antioxidant effects of betalain.
One definition of a conjugated system is:
“A system of connected p-orbitals with delocalized electrons in molecules with alternating single and multiple bonds.”
A nutritionist’s advice to “eat the rainbow” is really good advice. These colorful molecules can give up an electron without needing one back because the long conjugation system allows an oxidation-induced deficiency of electrons to be spread evenly among many atoms that are connected. These molecules are effective because they are large, but this is also a problem. The large size leads to problems with bioavailability, solubility, delivery and disposal of the oxidized waste products after they have been depleted.
What is so different about the naked electron? The naked electron travels through tissues and can act at a distance. To do this, the electron must be ejected from the atom that holds it with great force, the force we know as voltage. Voltage sounds scary, but humans regularly build up charges equivalent to 10,000 volts when they get a static charge that causes the shock of “zapping” a doorknob. When we deliver electrons from a Heliopatch’s specially treated magnesium core the voltage is a maximum of 4.75 volts, something that 4 AA batteries can surpass. Heliopatch only delivers a small amount of amperage, and here, the AA battery delivers 2.4 amps, which far outclasses the Heliopatch, which only delivers a couple of hundred microamps or 200 millionths (1/1,000,000) of an amp. To put that in perspective, the effect is just on the edge of perception for most people unless you put it on your tongue where it produces a pleasant flavor. The real difference between a battery and a Heliopatch is the destination of the electrons. In the battery, both an oxidizing and a reducing agent are supplied (the positive and negative ends), which means there is a “round trip ticket” for the electron that emerges from the battery. In a Heliopatch, the electron’s trip is strictly one-way. The electron goes into the skin, finds a free radical and then neutralizes it. The oxidized waste product of the reaction is a magnesium ion (Mg2+), which is commonly applied to skin as very concentrated forms such as magnesium oil (MgCl2). Heliopatch is the negative end (the anode) and the person is the positive end (the cathode), thus making the person one-half of the battery.
The naked electron can act at a distance because the body is composed of electrically conductive materials. There are many bodily fluids that make fair electrical conductors lymph, blood, saliva, sweat and tears; these all contain some level of salts which allow them to conduct electrons. There are other conductive components of the body that are just beginning to be characterized by science such as the extracellular matrix and the fluid paths within and around neurons.
The electrons from a Heliopatch come into the body and immediately diffuse, spreading out in a pattern that flows toward areas of inflammation and preferentially travel down the most electrically conductive paths. Instead of damaging the tissue the way that high current and voltage shocks do, these electrons support the normal functioning of neurons, muscles and other tissues by neutralizing harmful free radicals. If antioxidants from the diet are acting as hand to hand combatants, naked electrons act at a distance like bullets from a rifle. Because the quantity of electrons flowing is low and the paths they take are diffuse, these don’t disrupt the normal functions of muscles and organs. These act instead to “clean up” harmful free radicals that result from normal cellular metabolism as well as inflammatory dysfunction.
There are other interesting ways for electrons to move. One phenomenon is the solvated electron, which has been known since its discovery by Sir Humphry Davy in 1807. In the solutions that he and later researchers observed, the electron floats free of any molecular carrier. These solvated electrons act like the negative ion, so instead of the familiar sodium chloride (NaCl), which in solution is Na+ and Cl– dissolved in water you might have sodium electron (Nae–), which can actually be crystallized as a salt. When sodium metal is dissolved in ammonia the solvated electron produces a brilliant blue color of dissolved electron (e–). The solvated electron explains why it is easier to push electrons into a solution and charge it negative than it is to take electrons from a solution to charge it positive.
Once the electron is in you and past the resistive layer of the dry skin on the surface, there is a free flow from an area of low potential (near the patch) to areas of high potential (in working, stressed tissues). The electrons flow to the areas of most need (most positive electrical potential) that are close to the patch, and then work their way further out as the dangerous radicals in close proximity are mopped up. More patches at one time can mean more effective coverage. We have yet to find a level of overdose for electrons in general when administered in this way.
The naked electron does have some chemical limitations. The voltage available is not high enough to neutralize some radicals at great distances, if at all. A radical such as superoxide has a standard reducing potential of just +0.089V. This is not high enough on the standard reducing potential chart to make it attractive at a great distance to the electrons given by magnesium, which is at −2.37V compared to SHE (The Standard Hydrogen Electrode).
Fortunately, the body has a set of enzymes known as Superoxide Disumutase (SOD). These are very fast and efficient, allowing the superoxide to be converted to hydrogen peroxide (H2O2) the hydrogen peroxide can then be dealt with by enzymes such as catalase and glutathione peroxidase. We have chosen our source of free/naked electrons so that its voltage goes after the really damaging radicals that the body has no natural protection against, leaving the body’s natural system to handle weaker radicals that remain stable long enough for enzymatic stabilization and disposal.
A radical like the hydroxyl radical (•OH) has the highest reducing potential of any biological radical at +2.38V. The contrast between the naked electron and the hydroxyl radical provides +4.75 volts to work with. The naked electron in our patch acts at a distance, and this distance increases as the potency of the targeted radical increases. Hydroxyl radicals are an ideal toxic radical to neutralize by this method since there is no enzyme or natural defense beyond the antioxidants ingested from the diet to counter these radicals before they do damage.
Some radicals are important to proper biological function such as Nitric Oxide (•NO), this is a reducing radical important for dilation of blood vessels. Nitric oxide often does not make it across the synaptic cleft to the receptors in inflamed individuals. Fortunately, nitric oxide has a low standard reducing potential at -0.8V. We have found that the effect of the naked electron is actually preserving nitric oxide for the benefits it has during a workout where blood vessel dilation is very helpful.
Nitric oxide is a reducing radical. This means that it is unstable because it has an unpaired electron, but unlike the hydroxyl radical, which will take an electron, nitric oxide becomes stable by giving up an electron. When nitric oxide is released into the bloodstream or is emitted from a synapse, it is in a much more hazardous oxidizing environment than the reducing environment inside the cell. This means that oxidizing agents in the extracellular matrix and the lumen of the blood vessel can immediately attack nitric oxide, limiting of the duration of its action. This is naturally how the effects of nitric oxide are halted in the body, but in an inflamed individual, nitric oxide may not last long enough to have its proper impact upon physiology.
Athletes have been steadily increasing nitric oxide by using supplements and other supportive methods, but there may be a “too much of a good thing” problem as pointed out by O’Donnell and Freeman:
“Alternatively, if generated at elevated levels, for example, after inducible nitric oxide synthase expression in inflammation, •NO can be converted to prooxidant species, such as peroxynitrite (ONOO–) and nitrogen dioxide (•NO2), that can potentiate inflammatory injury to vascular cells.” (O’Donnell & Freeman, 2001)
Inflammatory injury to vascular cells is a serious problem and the main cause of vascular diseases such as atherosclerosis and heart attack. Leopold and Loscalzo describe the process of vascular damage by oxidizing radicals:
“In the vasculature, reactive oxidant species including reactive oxygen, nitrogen, or halogenating species, and thiyl, tyrosyl, or protein radicals, may oxidatively modify lipids and proteins with deleterious consequences for vascular function. These biologically active free radical and non-radical species may be produced by increased activation of oxidant-generating sources and/or decreased cellular antioxidant capacity. Once formed, these species may engage in reactions to yield more potent oxidants.”2 (Leopold & Loscalzo, 2010)
The highly mobile and rapidly reacting naked electrons in Heliopatch are directly attracted by the most potent radicals and oxidizing agents. Some oxidizing agents such as nitroperoxyl can travel through the body and suddenly become reactive radicals, but as soon as they do, they will draw the naked electron and be neutralized. This process of electrons flowing toward oxidizing radicals isn’t like aiming a gun, it is a much more passive process like the way that water flows downhill. This makes it immediately effective and incredibly potent no matter what form an oxidizing agent takes and what it does once it becomes a radical. The naked electron is a perfectly matched opposing force to the radicals, the faster and more powerful they are, the more rapidly they are neutralized and at a greater distance.
Some concerns have been raised about the nature of the electrochemical reactions and whether some electrons could react with an ion like sodium (Na+) or potassium (K+) to convert these into their elemental metallic compounds. This is an impossible chemical reaction because it does not satisfy the criteria for a spontaneous reaction. Some reactions have an abundance of energy that makes the chemistry happen spontaneously, like the interaction between magnesium metal and a hydroxyl radical. The total energy in this reaction is +4.75V as you can observe in figure 3.
1 http://circres.ahajournals.org/content/88/1/12.long
2 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2797369/
| 2OH•(aq) + 2e– | → | 2OH–(aq) | +2.38V | (inside the body) |
| Mg(s) | → | Mg2+(aq) + 2e– | +2.37V | (within the patch) |
| = | +4.75V |
Figure 3 – The reaction of hydroxyl radicals with magnesium metal to form hydroxide and magnesium ions. This reaction does not require direct collisions between the reactants.
This same equation series can be written to propose that magnesium instead will give its electron to a potassium ion. See the series of half-reactions below that add up to a negative voltage.
| 2K+•(aq) + 2e– | → | 2K(s) | +2.93V | (inside the body) |
| Mg(s) | → | Mg2+(aq) + 2e– | +2.37V | (within the patch) |
| = | +0.56V |
Figure 4 – The non-spontaneous reaction between magnesium metal and potassium ions to form potassium metal and magnesium ions. This reaction cannot occur due to the negative voltage.
When a chemical reaction is proposed and the total of the voltage from the reaction is negative, it means the reaction will not occur. When you see a negative voltage, this indicates that the opposite is more likely to occur and that if all of the reactions were reversed, the reaction would have a chance to occur spontaneously. Here the amount of voltage is very small, so if the situation were reversed to make potassium metal (K) the electron donor and a magnesium ion (Mg2+) the electron acceptor, the reaction could have a chance of occurring, but this would have a very small probability, perhaps only occurring at a very high temperature to increase the energy to make the reaction occur.
At Heliopatch, we have undertaken 3 years of careful study and experimentation to ensure the safety and efficacy of the Heliopatch which supports natural biological function. After many prototypes and rearrangements, we are happy to say that we have maximized the benefits and have minimized the downsides of inconvenience and skin irritation. We hope your experience of using Heliopatch is as elevating as ours.
Works Cited
Leopold, J., & Loscalzo, J. (2010). Oxidative Risk for Atherothrombotic Cardiovascular Disease. U.S. National Library of Medicine National Institutes of Health.
O’Donnell, V., & Freeman, B. (2001). Interactions Between Nitric Oxide and Lipid Oxidation Pathways. American Heart Association.

When committing to a fitness style diet plan, one of the first things you may be told is to start eating chicken. The baked-chicken-for-dinner-every-night stereotype

Every athlete has their idols. The person they saw on TV as a kid and thought, “That’s who I want to be.” For

