
Eat Your: Chicken
When committing to a fitness style diet plan, one of the first things you may be told is to start eating chicken. The baked-chicken-for-dinner-every-night stereotype


Antioxidant Supplementation- Effects on Exercise and Recovery
Antioxidant supplementation shows significant benefits for athletic performance during and after sustained physical activity. These correlations have been significant, reproducible and robust. One of the most studied antioxidants is quercetin, a flavonol found in many fruits, grains and vegetables, with the highest concentrations found in elderberries, blueberries and onions.
In one study of antioxidant supplementation in athletes (MacRae HS, 2006)¹ time trials (TT) with elite cyclists were conducted using a randomized, double-blind, cross-over study to test the effects of twice daily antioxidant supplement (AS) containing essential vitamins plus quercetin (FRS), and AS minus quercetin (FRS-Q) versus a baseline TT.
The results of this study showed an impressive increase of 9.39% on FRS compared to Baseline (P ≤ 0.01). Without the additional quercetin (FRS-Q) there was a 5.78% (P ≤ 0.05) increase in power. This showed both the positive effects of a traditional vitamin supplement (FRS-Q). In addition, the effects of this vitamin supplement were increased by 3.61% because of the extra antioxidant “boost” of quercetin (FRS).
These results are quite revealing, because they demonstrate that for these athletes performing at a high level and at their maximum endurance, there was an unmet need for antioxidant even when they were taking a regular vitamin supplement. Additional antioxidant in the form of quercetin improved their performance an additional 3.61%. This leads to questions such as, “How much antioxidant is enough.” and “How much more could be added before the effect plateaus and does not yield any additional increase in performance?” These are questions that only more extensive studies with varying dosage levels of antioxidants could answer. Given the high cost of these supplements and the unpleasantly high volumes of material that may need to be taken, there may be practical limitations other than the plateau level with regard to performance enhancement.
Potential Pharmacological Effects of Molecular Antioxidants
One problem encountered in any study of small molecule antioxidants is that these often have other pharmacological effects due to their shape and chemistry which allows them to interact with receptors. This makes them act more like drugs than nutritional supplements. Sometimes, it is the oxidized “used up” forms of these antioxidants that can exert drug-like effects, making their drug-like functions difficult or impossible to distinguish from their antioxidant functions. Quercetin provides an example of this mixed functionality. An oxidized derivative of quercetin known as dihydroquercetin or taxifolin antagonizes adenosine receptors in a manner similar to caffeine (Ji XD, 1996)². While most people consider caffeine a performance-enhancing drug, there are downsides to
1 http://www.ncbi.nlm.nih.gov/pubmed/17136942
2 http://www.ncbi.nlm.nih.gov/pubmed/8576921
having these drug effects. The problems of jitteriness, withdrawal headache and overstimulation can throw off a highly tuned performance. A just right dose of caffeine can be pushed into the realm of overdose when combined with other caffeine-like substances such as dihydroquercetin.
Quercetin has also been shown to increase mitochondrial density correlating to SIRT1 activation. Activated SIRT1 in turn activates PGC-1α by deacytylation. Increased levels of SIRT1 and PGC-1α coincided with increased levels of mitochondrial DNA after quercetin supplementation in mice (Davis JM, 2009)³. An increase in mitochondria numbers are quite beneficial to performance as mitochondria are the energy producers of the cell. Impressive results like these show increases in the numbers of mitochondria within brain and muscle, but there is still the lingering question about whether this arises from pure antioxidant activity, or pharmacological interaction with a receptor. It is possible that the antioxidant and/or the pharmacological effect could lead to an increase in the number of mitochondria. If the purely antioxidant action of quercetin increases the number of mitochondria, then any other antioxidant may fill the same role. If this is due to a specific receptor interaction, then other antioxidants would not necessarily provide the same benefit.
In order to determine what is happening and why, researchers often use combined feeding experiments to determine whether antioxidants with very different structures can produce the same results. These studies provide two antioxidants simultaneously and separately to determine if they have redundant or synergistic effects. Unfortunately, most of the antioxidants that could be used in combination with quercetin will have their own potential to activate receptors, and these activities may not yet be characterized; making it difficult to find a “pure” antioxidant.
Quercetin definitely exerts antioxidant effects and has been tested against free radicals. One study (Boyle SP, 2000)4 showed that increased plasma levels of quercetin resulting from a meal of onions was accompanied by increased resistance to DNA strand breakage in lymphocytes as well as decreased levels of some oxidative metabolites in the urine.
When we consider antioxidants, we rarely talk about their pharmacology. Because these are not thought of as drugs acting on particular receptors, it is difficult to track where they go, what they do, and what happens to them after they are oxidized. Basic questions about bioavailability and distribution into tissues are just now being investigated, and there are very few studies of how the oxidized antioxidants are removed from the body or their pharmacological activity. For instance, if the oxidized forms of quercetin had a different effect on receptors from the original quercetin, how would the results after strenuous exercise compare to the effects when there is low oxidative load and the majority of quercetin is excreted unchanged? Given the often
3 http://ajpregu.physiology.org/content/296/4/R1071.long
4 http://www.ncbi.nlm.nih.gov/pubmed/11131368?dopt=Abstract
undesirable effects that oxidation has upon pharmaceuticals, perhaps we should be more wary of the shape-shift our antioxidants undergo after they have been oxidized.
The closest a molecular antioxidant can be to being a “pure” antioxidant is hydrogen (H2) also known as elemental hydrogen or hydrogen gas. Hydrogen gas acts as an antioxidant by neutralizing hydroxyl radicals (OH•) and producing water (H2O). Hydrogen is particularly useful as an antioxidant research tool because it penetrates all organs and neutralizes only hydroxyl radicals. It is difficult, however, to quantify the bioavailability of hydrogen because it can exit the body through the skin and the quantity absorbed by any route is difficult to measure.
| H2(g) | 2H+(aq) + 2e– | 0.0V | ||
| 2OH•(aq) + 2e– | 2OH–(aq) | +2.38V | ||
|
H2O |
= |
+2.38V |
Figure 1– The reaction of hydroxyl radicals with hydrogen gas to form water. Reaction occurs only upon collision of H2 and OH• and always forms water (H2O).
Drinking hydrogen-rich water has been shown in numerous studies to decrease oxidative stress. In the following studies, the hydrogen enrichment is accomplished by placing magnesium metal in drinking water, and then drinking the resulting hydrogen-rich water.
Radiation therapy is one “pure” oxidative stress that hydrogen has been shown to counter. Two measures of blood antioxidant status were used. The first was derivatives of reactive oxidative metabolites (dROMs) the second was biological antioxidant power (BAP). A direct measure of the dROM level, showed a significant decrease in oxidized molecules in the patients drinking hydrogen enriched water. Similarly, BAP levels increased in these subjects reflecting increased serum antioxidant capacity (Kang K, 2011)5.
It is well understood that strenuous exercise increases free radical production in much the same way that redlining a car increases the emissions per mile. As conditions get further from optimum, oxygen becomes depleted and temperatures increase. This leads to enhanced oxidation and efficiency decreases. Since anaerobic metabolism inadequately maintains cellular energy states, ATP-stores are depleted and energy-dependent ion pumps fail. This causes mitochondrial leakage and dysfunction. In one study (Aoki K, 2012)6 of hydrogen-rich water consumed by high performing athletes the hydrogen-rich water imbibed before the test significantly decreased lactate levels in the
5 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3231938/
6 http://www.medicalgasresearch.com/content/2/1/12
blood compared to controls. The decrease in lactate was as much as 23% after 40 minutes of strenuous exercise. This reduction in lactate was accompanied by a more rapid return to baseline lactate levels after the exercise ended. The hydrogen-rich water also prevented post-exercise decrease of median frequency (MDF) after the initial strenuous exercise. MDF is an indicator of muscle fatigue and is especially important in sports where more than one “sprint” of physical activity is required to give the final outcome of the event.
The Downside of Hydrogen Enriched Water
The administration of hydrogen as H2 saturated water or as an inhaled gas is difficult to accomplish on the field and the half-life of hydrogen within the body is very low, especially with rapid respiration and high oxidative load that accompanies vigorous activity. Drinking hydrogen-saturated water can provide some prolonged release of the gas into the body, but the volume of water needed for significant improvement may lead to excessive liquid in an athlete’s stomach which detracts from athletic performance. The subjects of this study consumed 500ml of water the night before the test and 1000ml of the water the morning of the test. Consuming a liter of water prior to competition can lead to sloshing in the stomach.
Loading Prior to Training or Competition
Hydrogen is unusual among antioxidants in that there is no loading required and hydrogen consumed just before an event has a significant impact upon performance in a very short time. The downside is that it must be consumed close to the time of the event to have its maximal impact upon performance. By contrast, most antioxidant supplements such as quercetin require a supplementation regimen that is taken throughout training and competition. If the athlete allows this supplementation to lapse it can lead to decreased performance. This need for continuous supplementation can impose a significant financial burden on the athlete and may lead to poor compliance as the athletes forget a dose or become frustrated with regimes of supplements. They cannot miss a pill or they lose the benefits; even worse, they may experience the effects of withdrawal as the pharmacological effects of a supplement fade.
Antioxidants vs. Supplements vs. Drugs- Problems of Tolerance and Withdrawal
If a supplement’s effects are due to a receptor-mediated pharmacological interaction then there will inevitably be problems of tolerance even if the supplement is taken faithfully. Tolerance develops over time as the continual bombardment of a receptor leads to its down-regulation or up-regulation when the receptor is agonized or antagonized. We see tolerance and withdrawal effects even for a very benign receptor antagonist such as caffeine. Tolerance leads to higher amounts of the supplement being needed to achieve previously attainable results. Tolerance is the flip side of withdrawal; they are two aspects of the same mechanism. Tolerance counters the effect of the drug, while withdrawal is the opposite of the drug’s effects that are revealed when the drug’s “push” upon the receptor activity is suddenly withdrawn. Neither tolerance nor withdrawal are desirable, which is why many drugs provide only short-term gain and in the long term can cause more problems than they solve.
Route of Administration
Orally administered supplements such as pills have many troubling aspects because they interact with the food the athlete must also consume. Foods and beverages may absorb supplements and allow them to pass through the digestive system, making them less available. For example, calcium is a common “blocker” when it is co-consumed with supplements because it can lock these up in insoluble precipitates. On the other end of the spectrum, foods and beverages can sometimes speed up the absorption of supplements, causing too much to enter the system at once. For example, drinking a carbonated beverage along with supplements can cause stomach emptying and rapid absorption in the small intestine. This is a well understood pharmacological effect associated with champagne, where the same amount of alcohol consumed on an empty stomach leads to more inebriation with champagne than with wine. Since supplements are often taken at the beginning of a meal, it makes a difference when the carbonated beverage is consumed and how much. Rapid absorption of supplements can often be irritating to the stomach and the intestines, especially if there is no food consumed at the same time, not to mention the toll on the liver to process so much at one time.
Taking everything by mouth can be especially difficult when an athlete doesn’t feel hungry or thirsty. Often the anxiety of a performance will make it almost impossible to choke down pills and one bout of vomiting can throw the supplementation regime into disarray. Consuming a large quantity of pills or even hydrogen rich water before an event can be very difficult.
The Problem of Delivery and Waste Removal
When ingested supplements do make it into the blood, the products still have to be delivered to the brain, organs and muscles that need these and will utilize them. The process of absorption into the target tissue and the removal of the spent antioxidant by the venous blood can be a “traffic jam” at times, with a great number of molecules having to move in and out of tissues pushed to their peak of performance.
The route in from the digestive tract to the target organs such as brain and muscles is not a simple one. Once a supplement is liquefied in the stomach, the blood from the intestines that has absorbed the supplement must travel into the portal vein. This blood all goes to the liver, where the liver acts as a barrier for harmful substances that might otherwise cause problems, however, it also causes a considerable delay in the onset of effects and becomes the first user of the antioxidants in the supplement. The liver may consume the lion’s share of the antioxidant supplement, especially for antioxidant molecules that do not have a transporter to take them away. The liver has many enzymes that can change the structure of the drug and this may change its functionality significantly.
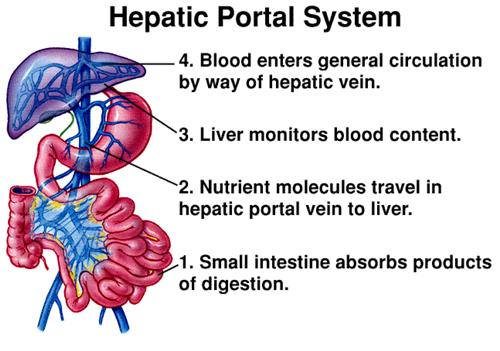
Figure 2 – The Hepatic Portal Vein and the route of ingested substances into circulation.
Once the antioxidants pass through the liver, they distribute throughout the body in a manner proportional to the blood flow in these areas. Because most antioxidants have either a lipophilic or hydrophilic nature, these partition either into fat or into the body water. This means that the distribution is destined to be uneven, with poor solubility and fleeting effects in target areas as the antioxidants accumulate in the parts of the body that can hold them.
The blood-brain barrier is another problematic impediment to antioxidant distribution. When the destination is the brain, many antioxidants cannot cross and will not remain near the brain. Because the brain has a very high level of energy and oxygen use and is a very dense, hot organ, the antioxidant need can be quite intense. The brain is actually a hot spot for oxidation and is the location where many symptoms of fatigue are the greatest.
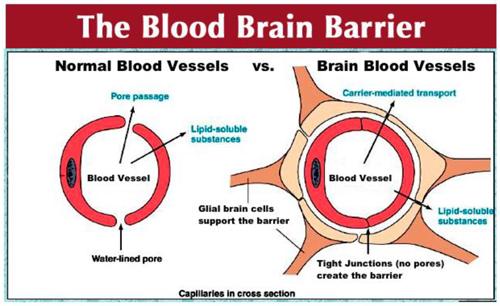
Figure 3 – The regulation of migration of antioxidants out of the bloodstream into the brain is much more complex in the brain.
How much do you take at once? How long will it last? Will it leave you hanging? How often will you have to “go”?
There is also the question of what quantity of supplement is practical to ingest at one time. Clearly, as endurance events grow longer or more intense, the supplements taken in the beginning of the event are used up before the end. By depending upon ingestion, there is an unfortunate “cliff’s edge” to fall off of as the supplement is used up and its waste products are eliminated into the blood, and then eventually to the bladder. Most people are familiar with the consequences of ingesting caffeine or alcohol; the body eliminates more urine when there are more wastes. This means that some supplements can lead to rapidly filled bladders and result in handicaps such as rapid dehydration and frequent stops.
What is needed- Immediate Effectiveness and Long-Term Efficacy
If an athlete were to have a supplement that isn’t taken orally, the pharmacology and half-life of the supplement would be much simpler and the athlete could get more consistent results. Additionally, topical administration to the target body part would allow much smaller amounts of the supplement to give greater results with less dilution. A more targeted supplement would free the athlete from long pre-event supplementation regimes and would have reduced interactions with food. The result is a much simpler, more normal life for the athlete.
Likewise, if the supplement were to release in response to the athlete’s needs while being lightweight, this would allow the supplementation to occur throughout the event without a need to resupply or drink large quantities of fluid. For events that take place over many hours, an as-needed slow release supplement would change how we view the use of antioxidants to not just enhance performance, but also to reduce the oxidative injury that an athlete must contend with.
The Heliopatch Solution We have developed a new type of antioxidant supplementation- direct electron donation. This system doesn’t send an antioxidant into the body to deliver an electron. Instead, it connects a potent source of electrons to the skin and these are transmitted into the body creating an electrochemical cell. These naked or free electrons are not like electricity and don’t have a chemical carrier; they flow within the body’s network of electrically conductive paths to the places where the free radicals are made. These electrons are most strongly attracted to potent free radicals such as hydroxyl radicals (OH•). The chemical equation below reveals the chemistry of two half-cell reactions- one happens on the skin surface where metallic magnesium corrodes, while the other happens deep inside the body.
| Mg(s) | → | Mg2+(aq) + 2e– | +2.37V | (within the patch) |
| 2OH•(aq) + 2e– | → | 2OH–(aq) | +2.38V | (Inside the body) |
| = | +4.75V |
Figure 4 – The reaction of hydroxyl radicals with magnesium metal to form hydroxide and magnesium ions. This reaction does not require direct collisions between the reactants.
The high innate voltage of this electrochemical reaction means that the anodic and cathodic reactions can occur at great distances from one another. Instead of requiring transport of the antioxidant through the bloodstream and adjacent to the free radical, the electrons can flow through tissues using electrically conductive routes that do not depend upon blood. When the free electron encounters the hydroxyl radical (OH•), it creates benign hydroxide (OH–) which increases pH; this alkalization provides a performance-enhancing effect of its own in addition to neutralization of the radical.
Magnesium Ions and Performance
Magnesium ions formed by the reaction can flow into the body and become part of the free magnesium pool. This free magnesium is rapidly exchanged and regulated within a much larger reservoir of magnesium that includes the magnesium in bones, lymph, cells and other tissues. Magnesium is needed in rather large quantities by human metabolism, and a majority of Americans have a dietary intake of magnesium below the RDA (supplements, 2013)7. An analysis of data from the National Health and Nutrition Examination Survey (NHANES) of 2005–2006 found that a majority of Americans of all ages ingest less magnesium from food than their respective EARs (Estimated Average Requirement) (Moshfegh A, 2009)8. Magnesium overdose is very uncommon and is mild, especially when it is not administered orally, which eliminates the laxative effect. These magnesium ions are nutrients which exert a positive influence on health
7 https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/
8 http://www.ars.usda.gov/SP2UserFiles/Place/80400530/pdf/0506/usual_nutrient_intake_vitD_ca_phos_mg_2005-06.pdf
and performance. This “coproduct” of electron supplementation contrasts with the oxidized forms of small molecule antioxidants, which must be degraded and eliminated from the body.
Hydroxyl Radicals, Hydroxide and the Alkalizing Effect
Hydroxide is considered by many to have positive effects on performance. It is known as an alkalizing effect, and alkaline substances have been used to enhance performance in athletics for many years. When the electrons flow into the person and attack a hydroxyl radical, this increases pH. Most metabolic processes result in acidic output such as CO2 (aerobic) or lactic acid (anaerobic). When you exercise more than your oxygen delivery can keep up with, the body utilizes anaerobic metabolism. A product of this metabolism is pyruvate, which becomes lactic acid; this acidity leads to the burning sensation in the muscle. This lactic acid moves from muscle to the blood, decreasing your blood pH.
Recent studies (Mueller SM, 2013)9 showed significant performance enhancement where average cycling time-to-exhaustion (Tlim) increased by 23.5% with NaHCO3 supplementation as compared to placebo. These results corroborate the performance enhancing results of ingesting sodium bicarbonate that have been observed since the seventies (Jones NL, 1977)10. In horse racing the use of buffering agents is banned, and limits have been set on the concentration of bicarbonate in blood samples taken before races (Amy M Gill, 2015)11.
In many examples of medical conditions that cause acidosis, the alkalization of the body has beneficial impacts. The use of enough potassium bicarbonate in the diet to neutralize the daily net acid load in postmenopausal women resulted in a significant increase in Insulin-like Growth Factor-1 (IGF-I). Low protein diets lead to a systematic decrease in many factors such as IGF-1; supplementation with bicarbonate increased the levels of IGF-1 from 95.9 ± 31.7 ng/ml to the same levels as the high protein group 136.4 ± 41.3 ng/ml, a statistically significant change of 40.5 ng/ml (Ceglia L, 2009)12. IGF-1 has many roles in the body; one well-documented role for this hormone is the anabolic increase in muscle mass (Velloso, 2008)13.
Bicarbonate supplementation works, but suffers from many of the same drawbacks as other supplements taken orally. Chronic ingestion of bicarbonate may cause intravascular volume expansion with resultant hyporeninemia and hypoaldosteronemia. These are conditions where low levels of renin and aldosterone dysregulate the water/salt balance in the body. Other side effects are gastrointestinal upsets such as vomiting and diarrhea (McNaughton, 1992)14.
Oral administration of bicarbonate has caused gastric rupture in at least 8 published case reports. Sodium bicarbonate can cause excess CO2 gas release when combined with gastric acid and can lead to gastric rupture which has a mortality rate as high as 65%. (Lazebnik N, 1986)15.
Clearly, the benefits of alkalizing the blood for athletic performance are significant, but the means employed to produce this effect through oral supplementation can carry some
9 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3623762/
10 http://www.ncbi.nlm.nih.gov/pubmed/24031
11 http://www.equiforce.com/bicarbonate-loading-in-horses.aspx
12 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2730228/
13 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2439518/
14 http://www.ncbi.nlm.nih.gov/pubmed/1331493
15 http://www.ncbi.nlm.nih.gov/pubmed/3020119
unacceptable risks which are not justified by the increase in performance. If the alkalinity was increased without the production of carbon dioxide, there would be no interference with the body’s normal signaling mechanisms. Likewise, without the evolution of CO2 gas the gruesome prospect of gastric rupture would also be avoided.
Heliopatch generates alkalinity electrochemically rather than through a direct input of an alkaline substance. Converting the dangerous hydroxyl radical (OH•) into hydroxide (OH–) turns the harmful into something beneficial. This is all without oral supplementation, ingestion or the evolution of gaseous CO2.
Works Cited
Aoki K, A. N. (2012). Pilot study: Effects of drinking hydrogen-rich water on muscle fatigue caused by acute exercise in elite athletes. July 12 2: 12. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3395574/
Boyle SP, D. V. (2000). Absorption and DNA protective effects of flavonoid glycosides from an onion meal. Eur J Nutr., Oct;39(5):213-23. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11131368?dopt=Abstract
Ceglia L, S. S.-H. (2009). Potassium Bicarbonate Attenuates the Urinary Nitrogen Excretion That Accompanies an Increase in Dietary Protein and May Promote Calcium Absorption. J Clin Endocrinol Metab., Feb; 94(2): 645–653. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2730228/
Davis JM, M. E. (2009). Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am J Physiol Regul Integr Comp Physiol., Apr;296(4):R1071-7. Retrieved from http://ajpregu.physiology.org/content/296/4/R1071.long
Ji XD, M. N. (1996). Interactions of flavonoids and other phytochemicals with adenosine receptors. J Med Chem., Feb 2;39(3):781-8. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8576921
Jones NL, S. J. (1977). Effect of pH on cardiorespiratory and metabolic responses to exercise. J Appl Physiol Respir Environ Exerc Physiol., Dec;43(6):959-64. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24031
Kang K, Y.-N. K.-B. (2011). Effects of drinking hydrogen-rich water on the quality of life of patients treated with radiotherapy for liver tumors. Med Gas Res., June 7 1: 11. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3231938/
Lazebnik N, I. A. (1986). Spontaneous rupture of the normal stomach after sodium bicarbonate ingestion. J Clin Gastroenterol., Aug;8(4):454-6. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3020119
MacRae HS, M. K. (2006). Dietary antioxidant supplementation combined with quercetin improves cycling time trial performance. Int J Sport Nutr Exerc Metab, Aug;16(4):405-19. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17136942
McNaughton, L. R. (1992). Bicarbonate ingestion: effects of dosage on 60 s cycle ergometry. J Sports Sci., Oct;10(5):415-23. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1331493
Mueller SM, G. S. (2013). Multiday acute sodium bicarbonate intake improves endurance capacity and reduces acidosis in men. J Int Soc Sports Nutr., Mar 26;10(1). Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3623762/Velloso, C. P. (2008). Regulation of muscle mass by growth hormone and IGF-I. Br J Pharmacol., Jun; 154(3): 557–568. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2439518/

When committing to a fitness style diet plan, one of the first things you may be told is to start eating chicken. The baked-chicken-for-dinner-every-night stereotype

Every athlete has their idols. The person they saw on TV as a kid and thought, “That’s who I want to be.” For

